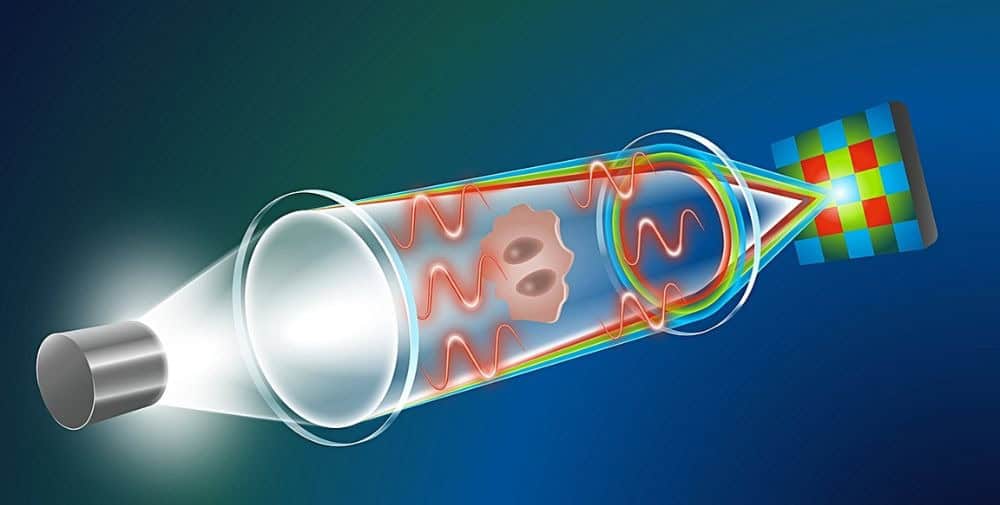
Stained or otherwise labeled biological samples provide valuable insights. However, a disadvantage for broad application in clinical diagnostics is that the process is time-consuming and requires expensive equipment and consumables. For this reason, research in recent years has increasingly focused on label-free microscopy methods such as quantitative phase microscopy. Here, it is not only the amount of light absorbed or scattered by the sample that is of interest. With the help of the scattering information, QPI also records how the sample shifts the phase of the light passing through it – a change that is directly related to its thickness, refractive index and other structural properties. QPI also requires quite expensive equipment, unlike computational QPI.
One of the best-known computational QPI approaches is based on solving the intensity transport equation (TIE). This differential equation enables the calculation of an image of the sample based on the recorded phase changes. The approach can be easily integrated into existing optical microscopy systems and provides high quality images. However, the TIE method often requires multiple exposures with different focus distances to eliminate artifacts. However, working with focus stacks is time-consuming and technically challenging, so this type of TIE-based QPI is often not practical in a clinical setting.
Chromatic aberration as a means to an end
“Our approach is based on similar principles as TIE, but requires only a single shot due to a clever combination of physical knowledge and generative AI,” explains Prof. Artur Yakimovich, head of a CASUS junior research group and lead author of the paper presented at the AAAI conference. The information about the phase shift caused by the biological sample does not come from additional images with different focus distances. Instead, chromatic aberration can be used to generate a focus stack from a single image. Most microscope lens systems cannot perfectly focus the wavelengths of (polychromatic) white light to a single convergence point – a disadvantage that only highly specialized lenses can compensate for. This means, for example, that red, green and blue (RGB) light have slightly different focus distances. “By detecting the phase shifts of these three wavelengths separately with a standard RGB detector, we can create a continuous focus stack that enables computer-aided QPI. So we turn the disadvantage into an advantage,” explains Yakimovich.
“A major challenge has to be solved if you want to make chromatic aberrations usable for QPI: the focus distance between red and blue light is very small,” says Gabriel della Maggiora, PhD student at CASUS and one of the two lead authors of the publication. In this case, the standard TIE solution does not provide any meaningful results. “Then we came up with the idea of using artificial intelligence. This idea proved to be decisive,” recalls della Maggiora. “After training a generative AI model with a freely accessible data set of 1.2 million images, it was able to determine the phase information despite the very limited data input.”
Testing the method on specific clinical samples
The team used a generative AI model for image enhancement that it had presented last spring: the Conditional Variational Diffusion Model (CVDM). It belongs to the diffusion models, a special group of generative AI models. The developers emphasize that training a CVDM requires significantly less computing power than training other diffusion models. The results are equivalent or even better. By using a CVDM strategy, della Maggiora and his colleagues were able to develop a new diffusion model that is suitable for quantitative data. With this model, they were finally able to realize computational QPI based on chromatic aberrations. For example, they validated their generative AI-based approach using a conventional brightfield microscope equipped with a commercially available color camera to capture microscopic images of real clinical samples. When analyzing red blood cells in a sample of human urine, the method was able to visualize the characteristic donut-shaped structure of these cells – a result that another established computational TIE-based method could not deliver. An additional advantage was the almost complete absence of cloud artifacts in the images calculated with the new QPI method.
The Yakimovich group “Machine Learning in Infection and Disease” is developing novel computational microscopy techniques that could be used directly in clinical applications. The potential uses, particularly in diagnostics, are enormous. The techniques used include generative AI. As generative AI is prone to so-called hallucinations, the group is focusing on minimizing these. The key approach here is to incorporate physical knowledge. As the example of AI-based quantitative phase microscopy shows, this approach holds great potential.
Publication
G. della Maggiora, L. A. Croquevielle, H. Horsley, T. Heinis, A. Yakimovich, Single Exposure Quantitative Phase Imaging with a Conventional Microscope using Diffusion Models, presented at the 39th Annual Conference on Artificial Intelligence by the Association for the Advancement of Artificial Intelligence (AAAI) and accepted for publication in the Proceedings of the 39th AAAI Conference on Artificial Intelligence
Contact
Dr. Artur Yakimovich | Junior Research Group Leader
Center for Advanced Systems Understanding (CASUS) at HZDR
E-mail: a.yakimovich@hzdr.de
– – – – – –
Further links
👉 www.hzdr.de
Photo: HZDR / blrck.de