If warning symbols light up in the car, a trip to the workshop is often necessary. There, a defective component may be identified as the cause and a replacement part pulled out of the drawer. What works for standard spare parts for cars is difficult to transfer to living beings. If you let your gaze wander through a busy pedestrian zone, it quickly becomes clear that no two people are the same. What applies to outward appearance also applies to the anatomy of the human body. The workshop that helps with toothache is called a dentist and the spare parts are not in the drawer there. The dentition, teeth and their arrangement are too different to replace a tooth one-to-one with standard products. In implant prosthetics, products that are tailored to the individual patient and provide them with the greatest possible benefit are suitable.
Additive manufacturing prints individual products
Additive manufacturing processes are often better suited to the production of patient-specific products than conventional processes, as they can produce different geometries without major additional effort thanks to their process sequence of layer-by-layer material application. In the case of abutments, for example, which are the connecting pieces between a dental implant and the visible dental prosthesis attached to it, the angles vary for the aesthetic and functional alignment of the dental prosthesis. Additive manufacturing processes such as “Laser Powder Bed Fusion with Metals” (PBF-LBM) produce several individual abutments cost-effectively in a single printing process.
Safety first!
What is feasible in terms of production technology does not necessarily have to be safe at the same time. With patient safety in focus, the aim is to reduce the risk to the patient to a minimum. The additive manufacturing process, with all its parameters, control variables and disturbance variables, is complex even for standard products, which means that suitable technical risk management must be used to meet customer and regulatory requirements (see ISO14971: Medical devices – Application of risk management to medical devices). For individual products, the verification of safe products is complex due to potentially deviating product properties caused by individual geometries.
No reason for uncertainty
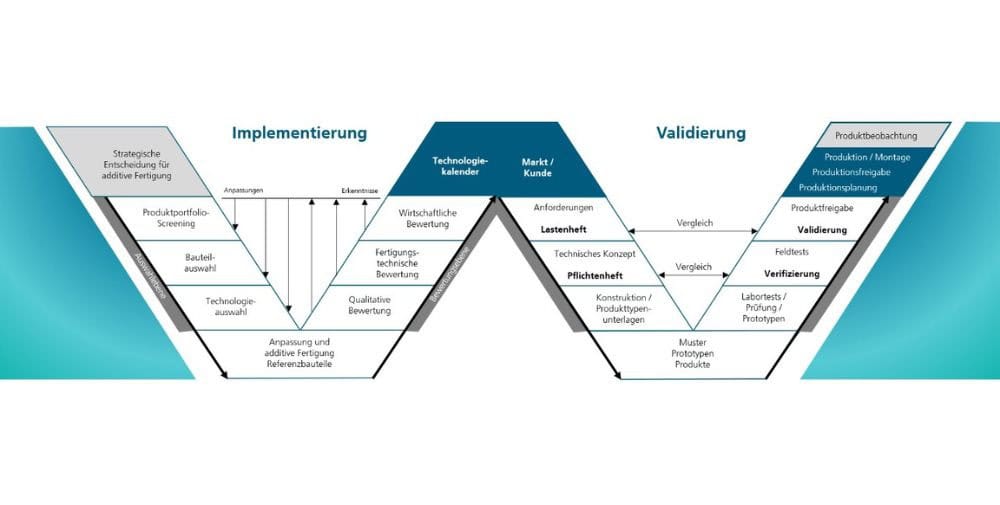
Experts at Fraunhofer IPA and the High Performance Center Mass Personalization have therefore developed a methodical approach for companies to implement additive manufacturing processes safely and cost-effectively and validate individual products, even in highly regulated industries. A guide to this can be found in an open access article. “The potential of additive manufacturing for individual products is huge. With a systematic approach, suitable products and technologies can be identified and their risks managed,” says one of the authors, Hajo Groneberg, categorizing the topic.
– – – – – –
Further links
👉 www.ipa.fraunhofer.de
Photo: Fraunhofer IPA/Graphic: Hajo Groneberg, Carolin Schulz